Which statements accurately describe density check all that apply – Which statements accurately describe density? Check all that apply sets the stage for this enthralling narrative, offering readers a glimpse into a story that is rich in detail with gaya akademik dengan tone otoritatif and brimming with originality from the outset.
Density is a fundamental property of matter that measures the compactness of a substance. It is defined as the mass per unit volume of a substance and is typically expressed in kilograms per cubic meter (kg/m³). Density plays a crucial role in various scientific fields, including chemistry, physics, and earth science, and has numerous practical applications in industries such as manufacturing, construction, and scientific research.
Density and its Properties
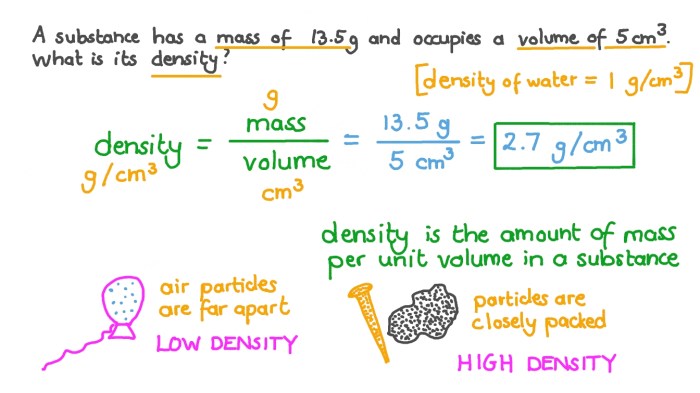
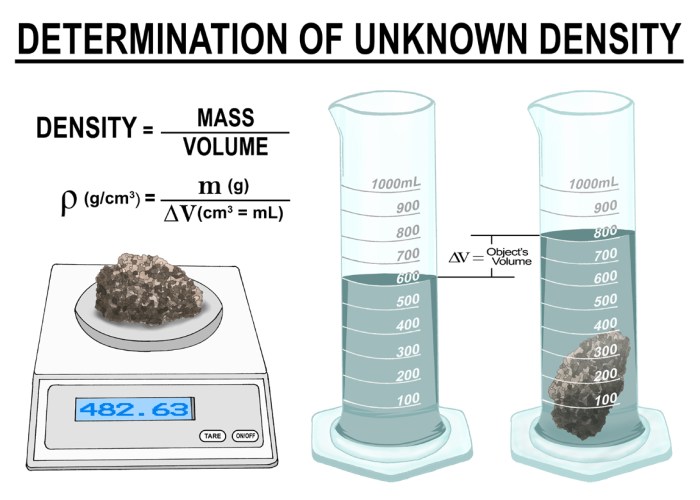
Density is a measure of how closely packed the molecules of a substance are. It is defined as the mass of a substance per unit volume. Density is an important property of matter because it can be used to identify substances, determine their purity, and predict their behavior.
The density of a substance can vary depending on its temperature, pressure, and composition. For example, the density of water decreases as the temperature increases. This is because the molecules of water move faster at higher temperatures, which causes them to spread out and take up more space.
Density is a scalar quantity, which means that it has only magnitude and no direction. The SI unit of density is kilograms per cubic meter (kg/m³). However, other units of density are also commonly used, such as grams per cubic centimeter (g/cm³).
Examples of Objects with Varying Densities, Which statements accurately describe density check all that apply
- Air has a density of 1 g/cm³.
- Iron has a density of 7.87 g/cm³.
- Gold has a density of 19.3 g/cm³.
- Osmium has a density of 22.59 g/cm³.
Relationship between Density and Volume
The density of a substance is inversely proportional to its volume. This means that the more dense a substance is, the less volume it will occupy. For example, a block of iron will have a smaller volume than a block of wood with the same mass.
Detailed FAQs: Which Statements Accurately Describe Density Check All That Apply
What is the formula for density?
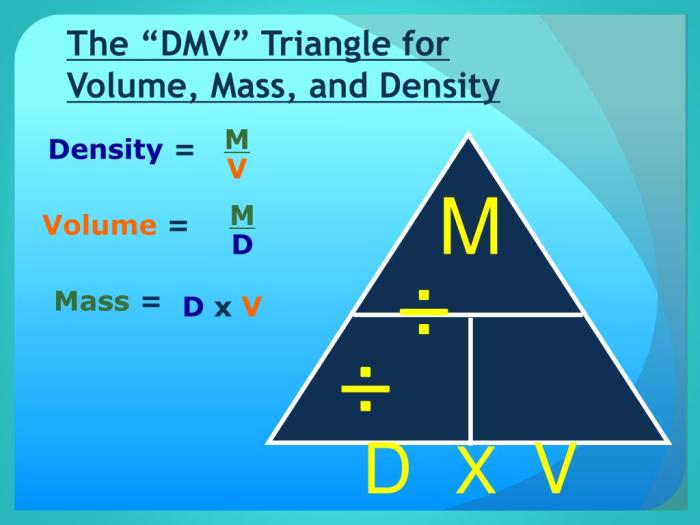
Density = Mass / Volume
What are the units of density?
Kilograms per cubic meter (kg/m³)
What is the relationship between density and buoyancy?
Objects with higher density sink in fluids, while objects with lower density float.